DNA origami
Building with genetic material
It sounds like paper handicraft, but it is in fact a well-established term in biochemistry: origami – just not with colourful paper squares, but rather with DNA, our genetic material. Scientists at Paderborn University are using DNA strands to build complex nanostructures that will ultimately be employed in biomedicine or help with surface patterning.
Background: DNA origami vs genetic engineering
‘Our research group is focusing on DNA origami – a relatively recent field of research. In doing so, we are seeking to investigate potential applications for this technique in the fields of biomedicine, biophysics, chemical biology and surface patterning’, explains Dr. Adrian Keller, head of the ‘Nanobiomaterials’ research group at Paderborn University’s Department of Chemistry. The research of Keller and his team falls under nanoscience, i.e. the investigation of atoms, molecules and structures in the nanometre range. To provide an idea of scale, one nanometre is one millionth of a millimetre. Although DNA is the focus of their work, this is not genetic engineering. Genetic engineers generally work on the isolation, analysis, targeted modification and transfer of an organism’s genes. The Paderborn researchers, on the other hand, are not modifying genes, but rather using DNA to build transport containers, lattice structures and other shapes.
In the laboratory: implementing the construction plan
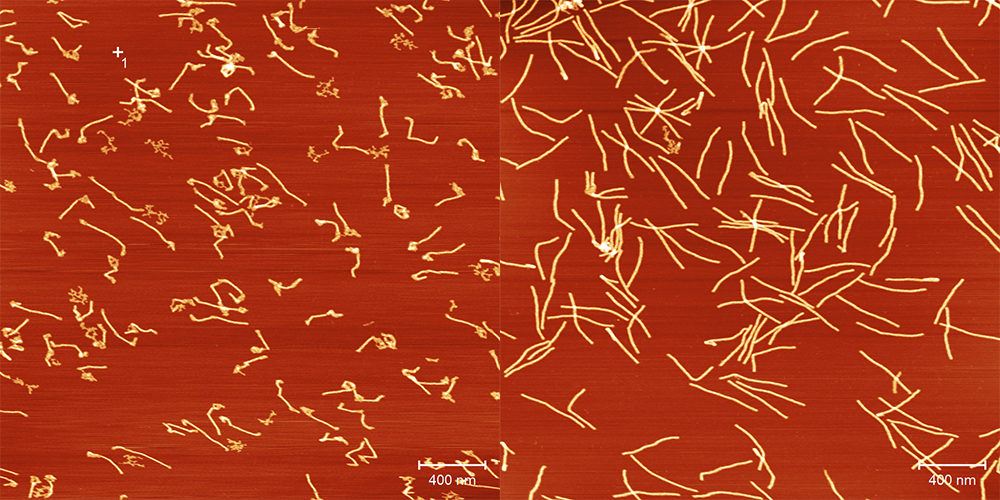
The Paderborn researchers start by using special computer software to check what the construction plan for their structure might specifically look like. Next, they order the synthetic DNA strands they need from specialist laboratories (see the info box for more information about the structure of DNA). Keller and his team then prepare these strands in the laboratory. Each origami always consists of one longer ‘scaffold’ strand and numerous short ‘staple’ strands: the short strands are selected to bind the longer strand in specific places, causing it to fold. To enable hydrogen bonds to form between the complementary bases, the researchers first heat the DNA to 80 degrees Celsius and then cool the mixture to room temperature in a controlled way. As a result, the desired shapes – which may be triangles, squares or more complex structures – in principle form by themselves: scientists describe this as DNA self-assembly. The shapes range from a few nanometres to hundreds of nanometres in size. The scientists then remove the excess DNA not integrated into the structure.
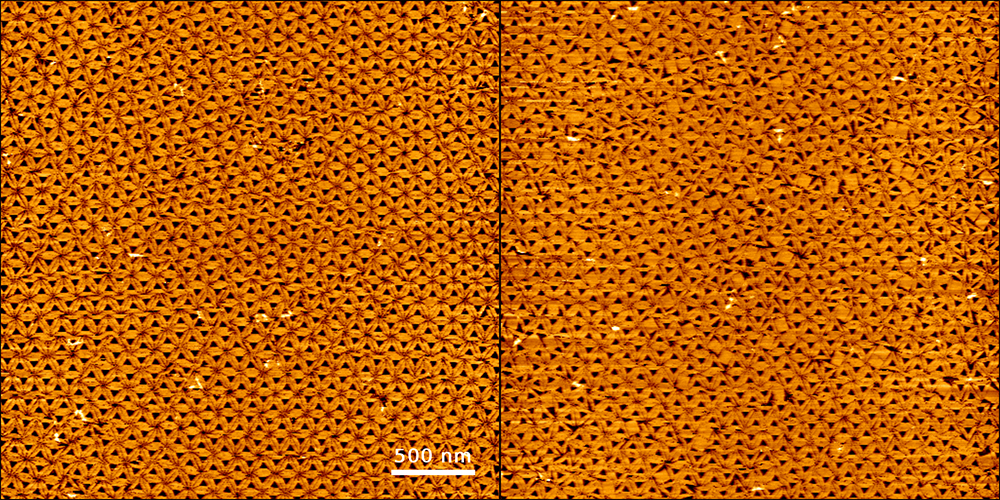
DNA origami are initially contained in a solution, but under some conditions can also spontaneously settle on surfaces. Researchers then examine them with a high-speed atomic force microscope to see how the DNA structures are arranged. ‘In an experiment where we were making triangular DNA structures, we were able to identify a hexagonal arrangement. The triangles connected with each other almost perfectly. If we add a few squares, we can see how this disrupts the arrangement of the triangles’, Keller explains.
View publications: https://doi.org/10.1007/s12274-020-2985-4 and https://doi.org/10.1039/d0nr01252a
Step one: fundamental research
As it currently stands, Keller and his colleagues are still conducting fundamental research. However, there are various areas of application where DNA origami could be used in a few years’ time. Since these DNA structures can bind to specific cells, they could be used in biomedicine to transport enzymes, fluorescent dyes (e.g. for diagnostic purposes) or chemotherapeutics. Pharmaceutical agents often need to be encapsulated in delivery systems that will take them safely to the part of the body where they need to act – and not release them any sooner, as this can be harmful depending on the substance. Drug delivery systems are essentially transport containers for therapeutic molecules. Given this, the scientists are specifically examining how origami structures could be efficiently loaded with the drug methylene blue, which is used in photodynamic therapy. Using a near-field infrared microscope that is accurate to 50 nanometres, it is possible to see how the drug docks to the DNA origami, for example.
View publication: https://doi.org/10.1039/D2NR02701A
DNA origami technology also offers a unique opportunity to arrange functional units, and in particular individual biomolecules such as proteins and nucleic acids, to a hugely precise level. This enables the creation of exact arrangements that can be used as platforms for studying individual molecules. High-resolution atomic force microscopes can be used to visualise individual proteins in DNA-origami-based arrangements like this, and thus distinguish between different events such as the formation or dissolution of protein complexes. Paderborn’s nanoscientists are currently using this technique to examine interactions between proteins and drug molecules, and to discover new protein inhibitors.
View publication: https://doi.org/10.1002/sstr.202000038
As part of a research project building on these topics, researchers will also be arranging antimicrobial molecules on DNA origami in a controlled way in order to increase their effectiveness against antibiotic-resistant germs. The project is being funded by Keller’s receipt of the Paderborn University Research Award.
Adrian Keller wins research award of Paderborn University 2022 for the work on DNA origami

The challenge: ensuring stability of newly created DNA structures
However, there are still a few challenges to overcome before DNA origami can actually be put to practical use. One of these is the stability of origami nanostructures. Under physiological conditions, i.e. as would be the case in the body, the folded DNA is broken down uncontrollably. Degradation is a desirable process in some cases (such as in drug delivery systems, where drugs need to be released in specific locations), but if the transport box opens too early, this can have fatal consequences. The researchers are therefore examining how different DNA origami nanostructures behave under the relevant environmental conditions found in the human body. ‘In our studies, we discovered that low magnesium concentrations and the presence of DNA-degrading enzymes are responsible for the destruction of DNA nanostructures’, Keller explains. Paderborn’s researchers have examined this in recent years. ‘We can now show that degradation in physiological media can be controlled using a specific design of the nanostructures. This means that we can tailor the stability of structures to a desired application in a targeted way.’ Keller is also seeking to use such designs to enable complex degradation profiles and thus the tailored release of drugs.
View publication: https://doi.org/10.1002/smll.202107393
Production: Gesa Seidel, Press, Communications and Marketing Office
Good to know
The abbreviation DNA stands for deoxyribonucleic acid. It stores the genetic information of living beings and DNA viruses. DNA itself consists of phosphates, sugar, and the four bases adenine, guanine, thymine and cytosine. These bases are linked together with hydrogen bonds. The bases fit in pairs: adenine and thymine pair together, as do guanine and cytosine. From this base pairing, DNA forms a double helix structure with two strands winding round each other in a spiral. When creating DNA origami, multiple individual strands are linked together via this base pairing in such a way that the resulting double helix folds into the desired shape. The strands are therefore essentially ‘interwoven’ like the threads in fabric.
Further information
The chair ‘Technical and Molecular Chemistry’ within the Department of Chemistry at Paderborn University is headed by Professor Dr.-Ing. Guido Grundmeier. It is organised into four research areas covering molecular adhesion, nanostructured surfaces and interfaces, the functionality and stability of polymer/oxide/metal interfaces, and in-situ optical spectroscopy at interfaces and (nano)biomaterials.